Live life at your Peak

Two major threats to long-term health:
FREE RADICALS & INFLAMMATION
They are the root cause of degenerative conditions and aging. Only the Peak Performance Pack has been shown to reduce free radicals and inflammation, letting your whole body perform at optimum levels.
Live life at your Peak
JUST ASK TIM.




Brain fog was lifted–Mandi Varnum



Doing things I never thought I would–Kerry Buxton


Live life at your Peak
JUST ASK KAREN.




Felt like I was in my 20's again–Marybeth Green



Blood pressure came down 10 points–Joe Hetzel


Inspired by NATURE
Patented Oligo® Technology mimics the way minerals are found in plants to deliver nutrition to the body that is far more absorbable than traditional minerals, while severely limiting free radical activity.



Delivers Nutrients Just Like Nature
Only Oligo® binds minerals to proteins and fibers (just like fruits and vegetables).
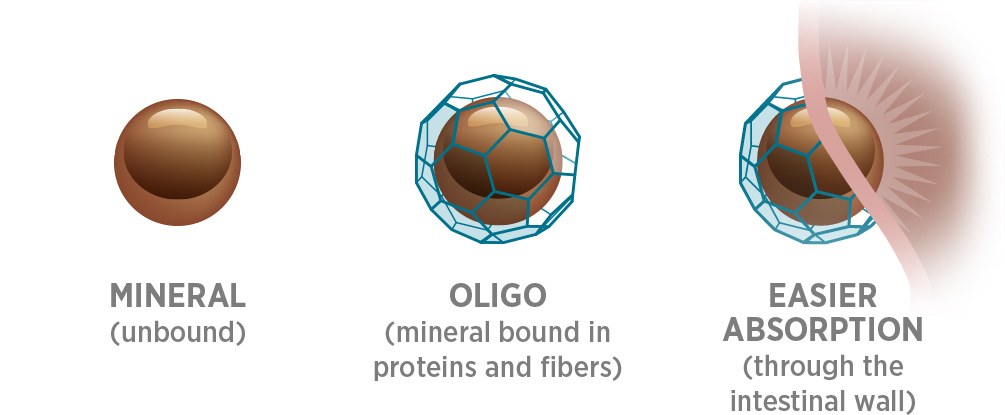
Patented Oligo Technology binds minerals the same way plants do to deliver nutrition that is far more absorbable than non-Oligo forms. This process also helps protect antioxidants by severely limiting free radical activity.
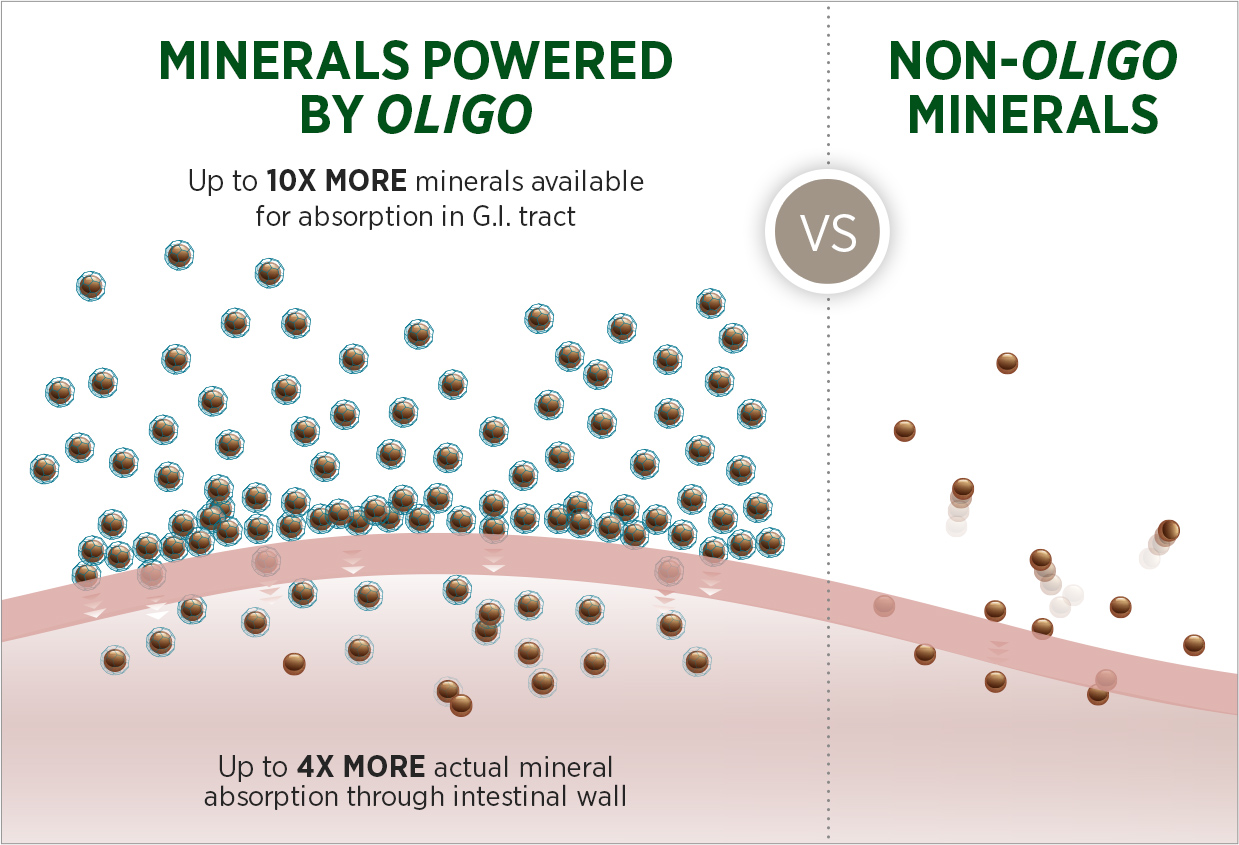
Proven by SCIENCE
Participants in human clinical studies showed improvement to 25 key health markers after taking Peak Performance—many results appeared within just 60 minutes. And health markers increased the longer they took it.















